Breaking the Cycle of Corporate Influence: The Need to Repeal PDUFA
Reforming the FDA’s Funding Model to Ensure Better, More Transparent Regulation
Repealing the Prescription Drug User Fee Act (PDUFA) is a proposal aimed at addressing the issue of corporate regulatory capture—a situation where regulatory agencies, which are supposed to serve the public interest, are unduly influenced by the industries they regulate. This is a critical issue in the context of pharmaceutical regulation because of the potential conflicts of interest that arise when the industry that is being regulated is also funding the agencies responsible for overseeing its safety and efficacy.
I have previously touched on corporate capture last year and you can read about it here:
Marketing Firm Providing PR for Moderna and Pfizer Also Staffed the CDC’s Vaccine Office
This is Why mRNA was Pushed as the Genetic "Vaccine" of Choice
Here’s a breakdown of the rationale behind repealing PDUFA and uncoupling money from regulatory agencies:
Understanding PDUFA:
The Prescription Drug User Fee Act (PDUFA) was passed in 1992 to expedite the approval process for new drugs by allowing the FDA (Food and Drug Administration) to collect fees from pharmaceutical companies. These fees help fund the FDA’s operations, particularly the drug review process.
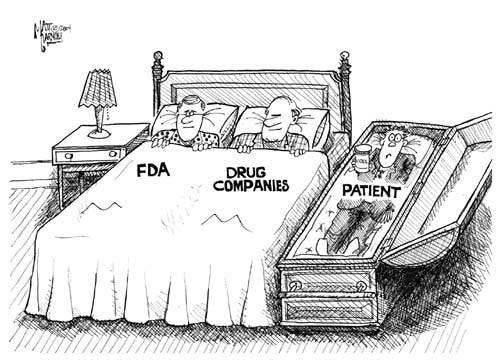
While PDUFA has been successful in speeding up drug approvals, it has also created a system where the pharmaceutical industry has a direct financial stake in the operations of the FDA.
The Problem of Corporate Regulatory Capture:
Conflict of Interest: Pharmaceutical companies pay fees (to the tune of millions of dollars) to the FDA for services, including drug reviews and regulatory approvals. This creates a situation where the regulatory agency could be influenced by the very companies it is meant to regulate, compromising its impartiality.
Compromised Public Trust: When the FDA depends on drug company fees for its funding, there’s a risk that its regulatory decisions might be swayed by the industry’s interests, rather than being purely based on scientific evidence and public health concerns. This undermines public trust in the FDA’s ability to protect consumers.
Pressure to Approve Drugs Quickly: Since pharmaceutical companies fund the regulatory process, there may be pressure for the FDA to expedite drug approvals, potentially at the expense of thorough safety evaluations. This is especially concerning for high-risk medications or treatments that could have severe side effects.
The Need for Independent Regulatory Oversight:
The primary goal of the FDA and other regulatory bodies should be to protect public health, not to cater to the financial interests of the pharmaceutical industry.
Uncoupling Funding: By repealing PDUFA, the FDA and other regulatory agencies could be funded directly by the federal government rather than relying on industry fees. This would help ensure that the regulatory process is independent, objective, and focused solely on public health, not influenced by the financial interests of the companies being regulated.
Ensuring Public Health Comes First: Without the financial influence of pharmaceutical companies, regulatory agencies would be better positioned to prioritize safety, efficacy, and long-term health outcomes when evaluating new drugs. This could lead to more rigorous, transparent, and ethical decision-making that serves the public interest.
Alternative Funding Mechanisms for Regulatory Agencies:
Public Funding: Regulatory agencies like the FDA could be funded through general tax revenue, ensuring their financial independence from the industries they regulate. This would allow these agencies to operate without the need for industry fees and would help eliminate the potential for conflicts of interest.
Accountability and Transparency: Shifting to public funding could make the FDA more accountable to the public and lawmakers rather than to pharmaceutical companies. This could increase transparency in the approval process, making it easier for the public to understand how decisions are made and ensuring that safety standards are consistently upheld.
Restoring Trust in Drug Approval Processes:
More Rigorous Safety Standards: Without the need to appease the pharmaceutical industry, the FDA would have more freedom to impose stricter safety and efficacy standards. This could reduce the likelihood of drugs being approved before their risks are fully understood.
Greater Scrutiny for High-Risk Drugs: The approval process could be more cautious and methodical for drugs with potentially serious side effects, ensuring that they undergo extensive testing and post-market monitoring before they are widely used.
Public Health Over Corporate Interests: The primary goal of the FDA and other regulatory bodies should be to protect public health, not to cater to the financial interests of the pharmaceutical industry. By repealing PDUFA, regulatory agencies could be more focused on the long-term health outcomes for patients, rather than being pressured to approve drugs quickly to satisfy industry demands.
Addressing the Larger Issue of Corporate Capture:
Wider Regulatory Reform: The issue of corporate capture extends beyond the pharmaceutical industry. Regulatory capture can occur in any sector where the entities being regulated are allowed to fund the agencies overseeing them. Addressing PDUFA could be part of a broader effort to reform regulatory agencies across different industries, ensuring that they operate independently and without undue influence from the industries they regulate.
Promoting a Balanced Approach: Repealing PDUFA would help balance the relationship between the government and the industries it regulates, making sure that regulatory agencies are truly working in the public interest rather than being swayed by financial incentives.
Potential Challenges:
Increased Government Spending: One challenge of repealing PDUFA is that it would require the government to allocate more resources to fund the FDA and other regulatory agencies. This could be met with resistance from lawmakers concerned about increased government spending. However, the long-term benefits of a more independent, efficient, and trustworthy regulatory system could outweigh the costs.
Slower Drug Approvals: Some argue that PDUFA has sped up the approval process for drugs, which has led to faster access to life-saving medications. However, this speed comes at the cost of thoroughness, and by uncoupling funding from industry, drug approvals might slow down—but this could be a positive development if it leads to better safety evaluations and fewer unsafe drugs entering the market.
Conclusion:
Repealing PDUFA and uncoupling industry funding from regulatory agencies is a necessary step to restore the integrity of the drug approval process. It would help eliminate the conflict of interest that arises when pharmaceutical companies financially support the agencies responsible for overseeing their products. By shifting to public funding, regulatory agencies like the FDA could operate independently, with a stronger focus on patient safety, efficacy, and long-term health outcomes. This reform would help to ensure that drug approvals are based on rigorous scientific evidence, rather than the financial interests of the pharmaceutical industry, ultimately promoting a healthier, more transparent healthcare system.





Beyond just its repeal it’s also how the safety studies are conducted. Are FDA employees former pharma. There needs to be complete separation ensured to provide true oversight and scientific rigor to drug approvals. Perhaps there could be reforms in the phasing of approval depending on the context of the IND etc, but many of these are already there. But the real question was the premise for why drugs need to be rapidly approved in the first place. Everytime I hear “to bring lifesaving drugs to more patients” == “we want to sell this fast.” Solid safety may rarely line up a fast approval, or result in approved drugs that are inconsistent like Aduhelm. These types of changes may also likely benefit the clinical diagnostic industry which is now under FDA purview.