Redefining Biosafety: How a Pause on Gain-of-Function Research Can Protect Public Health
How Temporary Research Pauses Can Strengthen Safety Protocols and Prevent Future Crises
Investigation into the origins of SARS-CoV-2 has sparked significant debate, with various theories, including the possibility of a laboratory leak. While definitive evidence will be difficult to obtain, the lack of clarity surrounding the origins of the virus underscores the potential risks associated with gain of function (GoF) research, particularly when working with highly contagious or harmful pathogens.
Gain of function research refers to experiments that aim to enhance the abilities of organisms (especially viruses or bacteria) by altering their genetic makeup to understand better disease transmission, pathogenesis, or resistance to therapies.
In 2014, a temporary pause on GoF research was implemented in the United States following concerns about safety related to flu virus studies and other pathogens. The U.S. government, through the NIH, issued a moratorium on certain GoF studies involving high-consequence pathogens (specifically influenza, SARS, and MERS viruses). A review was conducted to assess the risks and benefits, leading to the introduction of new guidelines and frameworks for the safe conduct of such research. This funding pause on GoF research was lifted in December of 2017.
If GoF research, especially involving coronaviruses or similar pathogens, contributed to the accidental release of SARS-CoV-2, this would highlight the inherent dangers of such research. This should have triggered another pause on GoF research pending investigation into the pandemic origins.
Here’s an outline of how a pause could be implemented on GoF research:
Pause Funding for Increased Scrutiny: A temporary freeze on funding for all GoF research would allow time for a comprehensive investigation into the origins of SARS-CoV-2. Such an investigation could explore whether GoF research, particularly in labs working on coronaviruses or other high-risk pathogens, may have played a role in the emergence of the virus. This pause would also signal to the scientific community and the public that the potential risks associated with this type of research are being taken seriously.
Incentivizing Cooperation: Freezing funding for GoF research could be a way to encourage greater cooperation in the investigation. Researchers, institutions, and governments involved in GoF research might be more inclined to cooperate if they see that a pause in funding is being used to ensure safety and accountability, rather than as a punitive measure.
Comprehensive Review Process: A thorough review of ongoing GoF and select agent research could ensure that it is being conducted under strict safety protocols and aligns with public health goals. This could include re-evaluating the necessity of such research, assessing its safety and ethical implications, and considering the potential for alternative approaches that carry less risk.
Evaluation by Independent Oversight Bodies: To maintain objectivity, the review process should be led by independent biosafety and biosecurity experts, rather than those who have a direct stake in continuing GoF research. This would ensure that the evaluation process is transparent, evidence-based, and free from conflicts of interest.
Implementing the Pause
To implement such a pause effectively and fairly, several steps would need to be taken:
Legislation or Executive Action: Freezing funding for GoF research may require an act of legislation or executive order, depending on the country. This would involve coordination between government agencies (such as the NIH, CDC, or WHO) and oversight bodies responsible for biosafety and biosecurity. The freeze should apply universally, to ensure that no institution or researcher is exempt.
Establishing Clear Criteria for Restarting Funding: To prevent indefinite halts, clear criteria would need to be established for resuming GoF research once the investigation is complete and any necessary reforms have been implemented. This might involve a set of guidelines for GoF research, stronger oversight mechanisms, and a demonstration of clear public health benefits.
Reassessing the Need for BSL-3 Facilities
Source: https://trdsf.com/blogs/news/biosafety-levels-comprehensive-guide
The rapid growth of biocontainment facilities since 2001, particularly in response to global threats like infectious diseases and bioterrorism, has indeed raised questions about their necessity, safety, and regulation. The biosafety level 3 (BSL-3) laboratories market expanded rapidly, driven by increasing global concerns about infectious diseases, bioterrorism threats, and the demand for advanced research facilities. The number of these labs grew by almost 10 percent, from 1,362 to 1,495, between 2008 and 2010 alone. With over 1,300 BSL-3 labs registered in the U.S. alone, and the increase in the number of these labs over the past two decades, there is a pressing need for careful reconsideration of the number and scope of these high-security facilities.
Source: https://asiatimes.com/2020/04/biosecurity-in-question-at-us-germ-labs/
Tightening access, narrowing the scope of research, and ensuring rigorous oversight are all critical steps in making sure that biosafety and biosecurity are maintained without unnecessary proliferation of high-risk laboratories. There should be a comprehensive review of the necessity of each BSL-3 facility currently in operation. This could involve assessing the risk, the potential benefits of the research being conducted, and whether safer alternatives (e.g., lower biosafety level labs or virtual models) might suffice. A reduction in the number of such facilities would minimize the potential for accidents and could help focus resources on more pressing research.
There also needs to be independent oversight and certification of biosafety containment facilities, particularly regarding the CDC’s role in certifying its own containment facilities.
Independent Oversight Committees
Establish an independent, third-party committee or body that is not affiliated with the institutions operating BSL3 and BSL4 labs. This committee could include experts in biosafety, public health, and laboratory management, who could regularly inspect and audit these facilities for compliance with best practices, standards, and regulations.
Global Collaboration and Training
I have contact with a fantastic biosafety trainer who travels the world and trains people to properly work within these facilities. This skilled biosafety trainer could be valuable in creating an effective and comprehensive certification or oversight framework for these critical facilities.
It might be beneficial to advocate for the establishment of global biosafety training standards. This could help in ensuring that professionals working in these high-security labs are consistently trained to a high standard and that facilities are adhering to international safety protocols.
Clear Certification Protocols
Push for the creation of a clear and transparent certification protocol for BSL3 and BSL4 labs, potentially spearheaded by independent experts. This could involve regular, surprise inspections, certification requirements for personnel, and a set of universally recognized best practices that all containment facilities must follow.
International Collaboration on Standards
Advocate for stronger international cooperation to establish and uphold standards for biosafety and biosecurity. This would ensure that high-risk research is being conducted in facilities that meet globally recognized safety and ethical standards and would create more uniformity in the regulations governing these types of labs across borders.
Conclusion
Pausing GoF research requires a multi-pronged approach that includes regulatory oversight, scientific review, and transparent decision-making. It is necessary to balance the scientific potential of such research with the need to safeguard public health and national security. This pause should be seen as an opportunity to enhance biosafety, build better systems for monitoring risks, and ensure that when GoF research does proceed, it is done responsibly and safely.




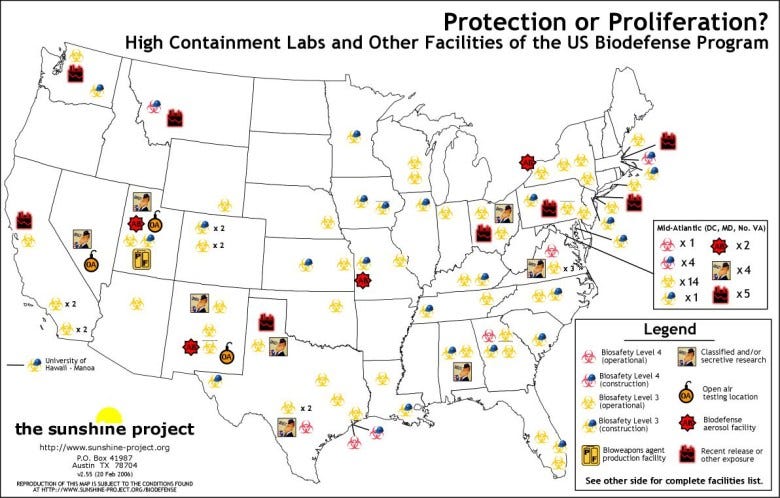
On one side GoF helps us understand zoonosis and potential ways that selection-induced strains can be transmitted and infect humans. I could even understand how surveillance of hot zones where it happens in the world could make sense. HOWEVER the risk for this type of research to go awry without extremely safe precautions and rigorous protocols is much too great to continue without an enormous PAUSE as Jennifer suggests! Not to mention it is also possible to be weaponized. Where in the world is this work being done…this needs to be explored. The fact that we had/have research facilities funded that do this work requires a complete shutdown before moving ahead….almost treat it like you are inspecting a nuclear power facility etc. Good post Jennifer.
Covid release was planned NOT an accident.