Should You Get the Shingles Vaccine? A Science-Based Look at Risk and Benefit
Breaking Down the Facts to Help You Decide
Many people have asked me whether or not they should get the shingles vaccine.
Disclaimer: I am not a licensed healthcare provider or medical professional. I do not offer medical advice. For any medical concerns or questions, please consult a qualified healthcare professional.
Perspective on Vaccination:
Vaccine decisions should be guided by a risk-benefit analysis. This includes evaluating the potential adverse effects associated with a specific vaccine, the likelihood and magnitude of its protective benefits (e.g., prevention of infection or mitigation of disease severity), and any off-target effects—whether beneficial or detrimental. Consideration should also be given to individual risk of exposure to the pathogen, as well as the possible clinical outcomes of infection.
It is important to recognize that not all individuals require vaccination against every pathogen. Variability in host factors—including age, underlying health conditions, occupational or environmental exposures, and immune status—can influence both the risk of adverse events and the magnitude of benefit derived from vaccination.
Shingles Explained: Causes, Complications, and Care
People get shingles when the alpha herpesvirus varicella-zoster virus (VZV), which causes chickenpox, reactivates in their bodies after they have already had chickenpox. During the primary infection (chickenpox), VZV migrates to the central nervous system, where it establishes latency in sensory neurons of cranial and dorsal root ganglia. More than 99% of Americans born before 1980 had chickenpox, even if they don't remember it.
About 1 in 3 people in the United States will develop shingles in their life. Children can have shingles, but it is not common. The risk of having shingles increases as people get older or if you have a weakened immune system. VZV reactivates due to stress, when taking immunosuppressant drugs (ie. steroids), or due to medical conditions that can cause immune suppression (ie. HIV).
In addition, mRNA COVID injections have been associated with an increase risk of shingles. There are multiple additional case reports of herpes zoster reactivation following COVID-19 vaccination in the literature. In 2021 a systematic review, identified 91 cases of VZV reactivation occurring an average of 5.8 days following mRNA vaccination. A large cohort study in California observed an increased risk of shingles of approximately 10% in the 90 days following the second dose of the primary series of the mRNA COVID-19 genetic vaccines.
Symptoms of shingles
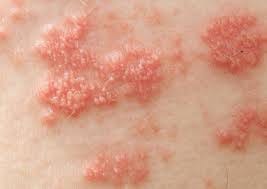
People with shingles most commonly have a rash around the left or right side of the body. The rash is usually painful, itchy, or tingly. Shingles generally lasts between 2 and 6 weeks. Other symptoms such as fever, malaise, and headache may be present.
Source: https://www.roselandsdoctors.com.au/understanding-shingles-symptoms-treatment-and-prevention/
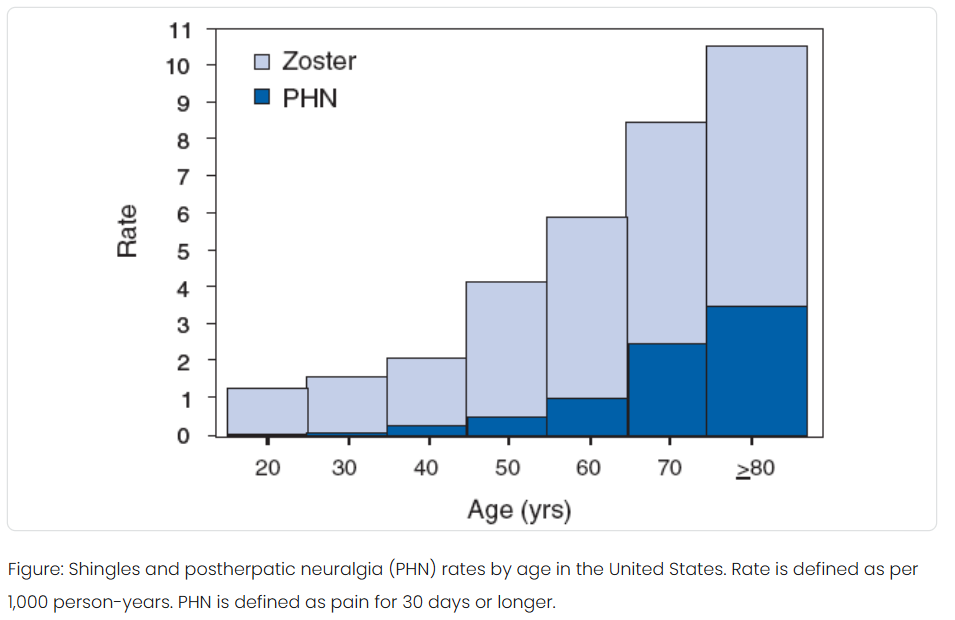
The most common complication of shingles is severe pain in the area where the shingles rash occurred. This is known as postherpetic neuralgia, or PHN persisting for 90 days or more after the onset of the rash. PHN develops as a consequence of the nerve damage caused by the reactivation of VZV in the ganglia. Approximately 10% to 18% of people with herpes zoster will get PHN. The risk of PHN also increases with age.
Source: https://www.cdc.gov/shingles/data-research/index.html
Is shingles contagious?
You cannot get shingles from someone who has shingles.
You can get chickenpox from someone who has shingles if you never had chickenpox or never got the chickenpox vaccine. You could then develop shingles later in life.
Most people who have shingles only have it one time. However, you can have shingles more than once.
Possible outcomes of shingles
Shingles isn’t considered a dangerous health condition and a majority of people who get shingles recover and resume their usual activities within a few weeks.
Up to 4% of people with shingles are hospitalized for complications, with older adults and those with weakened immune systems being more likely to be hospitalized. About 30% of people in the hospital for shingles have a weakened or suppressed immune system.
While shingles can be debilitating and cause severe pain, death from shingles is rare, Fewer than 100 people die from shingles each year. Almost all shingles deaths are in older adults or people with compromised immune systems. The risk of death from shingles increases with age and is highest in individuals over 80 years old.
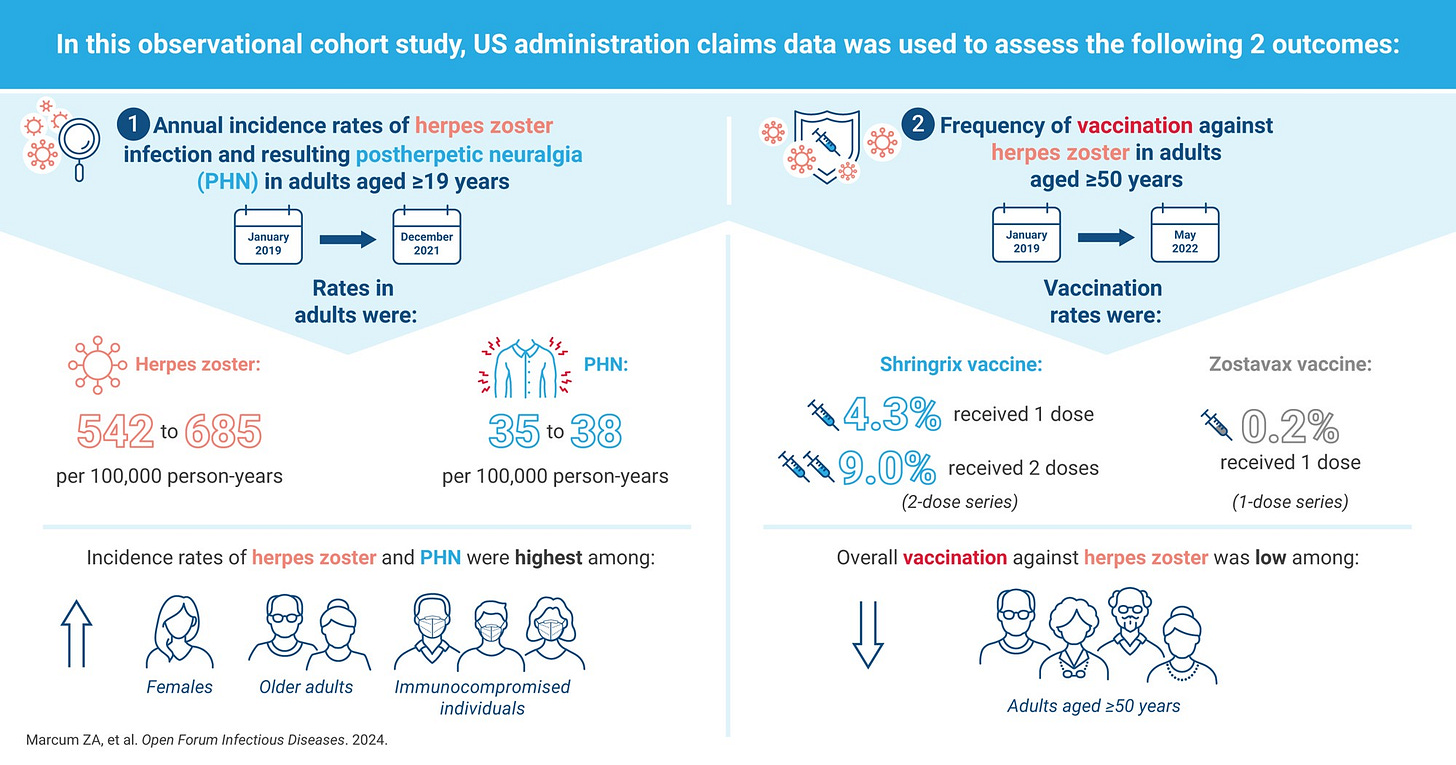
Source: https://academic.oup.com/ofid/article/11/5/ofae211/7646466
If left untreated, some complications of shingles can be fatal. Pneumonia, encephalitis, stroke, and bacterial infections can cause your body to go into shock or sepsis.
Treatment for shingles
Shingles treatment focuses on reducing pain, promoting healing, and preventing complications. Early treatment with prescription antiviral drugs such as acyclovir (Zovirax), may speed healing and lower your risk of complications. These drugs are most effective when started within 72 hours of the rash appearing to prevent complications like postherpetic neuralgia (PHN).
Pain management is crucial, and can involve over-the-counter pain relievers, topical treatments like calamine lotion, or prescription medications like corticosteroids, antidepressants or anticonvulsants. Additionally, cool compresses and loose, breathable clothing can help soothe the skin and minimize irritation.
Stress can exacerbate shingles symptoms, so it's important to manage stress through relaxation techniques or other methods.
Risks v. Benefits of the Shingles Vaccine
The first shingles vaccine, known as Zostavax, was approved by the FDA in 2006. It was a live, attenuated vaccine that contained a weakened form of the virus that causes shingles. Zostavax was discontinued in the United States in 2020 primarily because it was less effective and its protection waned over time compared to the newer Shingrix vaccine. A recent study showed that effectiveness of Zostavax against shingles in the first year (67%), dropped to 50% the second year, 27% the eighth year, and 15% after 10 years.
The main reason Zostavax was ultimately discontinued was because it had more contraindications and potential risks including the potential to cause death. Other side effects it caused were blindness, chickenpox, nerve damage, and paralysis.
Shingrix, a recombinant zoster vaccine (RZV), received its first marketing authorization in October 2017 to prevent herpes zoster and its complications in older adults.
Administration
It is recommended that adults aged 50 and older, and those 19 and older who are immunocompromised should get Shingrix. This includes individuals who have previously had shingles or the Zostavax vaccine.
Shingrix is a 2-dose vaccine series, typically given intramuscularly 2 to 6 months apart, that “boosts” the immune system to prevent the VZV from reactivating.
Ingredients
Shingrix consists of a recombinant form of the glycoprotein E (gE) antigen (50 ug) of the varicella zoster virus (VZV) combined with an adjuvant system called AS01B.
VZV gE is the most abundant envelope glycoprotein, predominantly expressed on the surface of virus-infected cells. The VZV gE protein plays a critical role in virus infectivity since it is involved in virus entry and cell-to-cell spread. The recombinant protein is produced in Chinese hamster ovary (CHO ) cells that were genetically modified to express the VZV gE gene.
AS01B is comprised of two “immune enhancers”:
3-O-desacyl-4'-monophosphoryl lipid A (MPL, 50ug), a chemically detoxified form of the lipopolysaccharide derived from the Gram-negative bacterium Salmonella minnesota
QS-21 (50ug), a saponin (triterpene glycoside) purified from the of the South American tree Quillaja saponaria Molina
MPL and QS-21 are combined in a liposomal formulation consisting of dioleoyl phosphatidylcholine (DOPC) and cholesterol in phosphate-buffered saline solution.
Each dose also contains 20 mg of sucrose (as a stabilizer), 4.385 mg of sodium chloride, 1 mg of DOPC, 0.54 mg of potassium dihydrogen phosphate, 0.25 mg of cholesterol, 0.160 mg of sodium dihydrogen phosphate dihydrate, 0.15 mg of disodium phosphate anhydrous, 0.116 mg of dipotassium phosphate, and 0.08 mg of polysorbate 80.
AS01B is a vaccine adjuvant that enhances immune responses but can also cause reactogenicity, meaning a more pronounced inflammatory response at the injection site. This manifests as pain, redness, swelling, and itching, according to the CDC. Systemic symptoms like fever, fatigue, and muscle aches are also more common.
Adverse and severe adverse events following Shingrix vaccination
Clinical Trial Data
Overall, 17,041 adults aged 50 years and older received at least 1 dose of SHINGRIX in 17 clinical studies. The “safety” of Shingrix was evaluated by pooling data from 2 placebo-controlled clinical studies (Studies 1 and 2) involving 29,305 subjects aged 50 years and older who received at least 1 dose of Shingrix (n = 14,645) or saline placebo (n = 14,660) administered according to a 0- and 2-month schedule.
In healthy subjects, solicited local and general adverse reactions were collected using standardized diary cards for 7 days following each vaccine dose or placebo. Adverse reactions with a median duration of 2 to 3 days following administration of Shingrix (both doses combined) were pain, redness, and swelling; and myalgia, fatigue, headache, shivering, fever, and gastrointestinal symptoms.
Unsolicited adverse events that occurred within 30 days following each vaccination included chills, injection site pruritus, malaise, arthralgia, nausea, and dizziness. Gout (including gouty arthritis) was reported by 0.18% (n = 27) versus 0.05% (n = 8) of subjects who received Shingrix or placebo.
Optic ischemic neuropathy (optic nerve damage) was reported in 3 subjects (0.02%) who received Shingrix (all within 50 days after vaccination) and 0 subjects who received placebo. From the first administered dose up to 30 days post-last vaccination, deaths were reported for 0.04% of subjects who received Shingrix.
Adverse Events Reported After Approval
The following adverse reactions have been identified during post-approval use of Shingrix: decreased mobility of the injected arm which may persist for 1 or more weeks; hypersensitivity reactions, including angioedema, rash, and urticaria and Guillain-Barré syndrome. In a post-marketing observational study, an increased risk of GBS was observed during the 42 days following vaccination with Shingrix.
Some individuals have died within a period of time after receiving Shingrix. In the initial post-marketing surveillance period (October 2017-June 2018), the CDC and FDA reported 7 confirmed deaths after Shingrix vaccination.
A recent study on the searched for and analyzed adverse event (AE) reports of Shingrix submitted to U.S. VAERS between October 20, 2017 and April 26, 2024. During the study period, 66,849 reports specifically related to Shingrix were received by VAERS. Most reports were classified as non-serious (97.3 %). The most commonly reported AEs included injection site reactions, pyrexia, chills, headache and fatigue. The median time-to-onset after vaccination of Shingrix vaccine from the vaccine adverse event reporting system was 1 day in all cohort groups over 50 years old.
As of April 2024, 86 deaths were reported following RZV (Recombinant Zoster Vaccine, which includes Shingrix) vaccination. All death reports occurred in individuals who received Shingrix as a standalone vaccine, with a median age of 74 years (range: 50–94 years) and a median time from vaccination to death of 1 day (range: 0–238 days). In addition to cardiovascular events and falls, Guillain-Barre syndrome was the most common cause of death.
How long does immunity from Shingrix last?
While Shingrix is highly effective, its protection may not last for your entire life. Immunity from the vaccine lessens over time, but it does so very gradually. Studies have shown that among people age 70 and older, Shingrix provides at least 85% protection against shingles for up to four years after completing the two-dose series, and remains high for at least five to maybe seven years. A study by the vaccine manufacturer, GSK, suggests Shingrix had nearly 80% (79.7%) efficacy in participants age 50 and older up to 11 years after vaccination.
“All vaccines are most effective just after they have been given,” Linda Yancey, MD, an infectious disease specialist at Memorial Hermann Health System in Houston said. “There is no one answer to the question of how long a vaccine is effective. It is going to vary from person to person.”
Until further research suggests otherwise, older adults generally only need to get the two-dose Shingrix series once. No Shingrix vaccine booster is currently available.
SUMMARY OF FACTS:
Shingles
If a person has had chickenpox then the virus that causes shingles is already in them.
Shingles isn’t a serious condition for most people who get it.
Within 2 to 4 weeks, the shingles rash fades and people resume normal activity.
Prescription medication, resting, and drinking plenty of water can help you heal faster.
Shingrix
Shingrix is a recombinant vaccine which contains a bacterial toxin to augment the immune response. In clinical trials, subjects were followed for only 30 days after each vaccine dose and several severe adverse events were reported in the vaccine group which included optic nerve damage and death.
In a post-marketing observational study, an increased risk of Guillain-Barré Syndrome was observed during the 42 days following the first dose of Shingrix.
Deaths were reported after administration of Shingrix in post-marketing surveillance period as well as through VAERS.
While Shingrix reduced the incidence of shingles, immunity following vaccination is not long-lasting and lessens over time.
Is the Shingrix “vaccine” safer than the shingles?
Considering the outlined risks of shingles and the associated benefits and potential side effects of the shingles “vaccine”, you are now prepared to make a well-informed decision regarding the Shingrix vaccination.





I’m a Pharm. D. I vote NO (to most vaccines)
Excellent summary and fair balanced information. As a clinical pharmacist and former medical researcher I appreciate your style. While I give and often recommend Shingrix I have refused it for myself. I lead a very low carb antiinflammatory lifestyle and find the less pharma I put in the less I have to detox from. Thanks again! I will be quoting your article with my patients so they can make an informed personal decison.