Important Update: Veterinarians Notified by FDA About Adverse Events Related to Librela
Agency reviewed thousands of cases of side effects, including deaths, reported for dogs that took Librela
FDA warns veterinarians about dangers of Librela
The other day, the FDA issued a letter warning veterinarians of adverse events related to administration of the monoclonal antibody therapy, Librela (bedinvetmab).
Source: https://www.wsj.com/health/pharma/fda-warns-about-dangers-of-dog-pain-drug-61b1dd63
Dear Veterinarian,
The U.S. Food and Drug Administration’s Center for Veterinary Medicine has completed an evaluation of adverse events reported in dogs of various ages treated with Librela (bedinvetmab injection). The adverse events identified and analyzed include: ataxia, seizures, other neurologic signs, including but not limited to, paresis, recumbency, urinary incontinence; polyuria, and polydipsia. In some cases, death (including euthanasia) was reported as an outcome of these adverse events. The FDA is making available reports containing summaries of clinical signs reported for Librela in the CVM FOIA Electronic Reading Room.
As of April 18, 2024, the CVM’s database contained 3,674 cases linked to Librela, reported through March 31, 2024. A number of the most frequently reported adverse signs, including the most common one—ataxia, which has been observed in 17% of cases—are not currently listed on the product labeling. Other frequently reported but unlabeled signs include polydipsia, death, diarrhea, muscle weakness, convulsions, recumbency, and paresis.
Many dogs began showing concerning clinical signs after their initial Librela dose. Two-thirds of the cases assessed reported signs occurring within the first week of administration, with 30% of cases showing signs within the first day.
Based on the evaluation and analysis of these reports, the FDA has recommended adding a Post-Approval Experience section to the Librela label. However, the FDA Center for Veterinary Medicine does not currently have the authority to mandate safety-related labeling changes.
Zoetis still contends Librela is “safe and effective”
Source: https://todaysveterinarybusiness.com/zoetis-librela-fda-121624/
In a response by the drug maker to the FDA letter, Zoetis released the following:
“We continue to have the utmost confidence in the safety and efficacy of Librela. Since launching in Europe over three years ago, Librela has been used effectively with millions of dogs suffering from osteoarthritis pain. With more than 21 million doses distributed globally, no individual adverse event sign is reported at a rate higher than rare, as defined by the European Medicines Agency (EMA) as <10 per 10,000 treated animals; 1 dose = 1 treated animal).”
Zoetis stands firmly behind the safety of Librela and its ability to provide relief for dogs suffering from osteoarthritis pain.
In the analysis, 458 (12.6%) cases of death associated with Librela were reported to the FDA. As a pet owner, I believe the suffering or loss of even one pet is too many. The fact that such serious adverse events have been documented raises important questions about the safety of this drug, especially when we consider the emotional bond we share with our pets. No amount of treatment should come at the cost of a pet's life or well-being, and it is crucial for both veterinarians and pet owners to be fully informed about the potential risks.
Zoetis puts profits above pets
A Fortune 500 company, Zoetis generated revenue of $8.5 billion in 2023 with approximately 14,100 employees. The company reported revenue of $2.4 billion for the third quarter of 2024, an increase of 11% compared with the third quarter of 2023. Last week, the board approved a 16% payment increase. The dividend will be paid on Tuesday, March 4, 2025, to all holders of record of the Company’s common stock as of the close of business on Tuesday, January 21, 2025.
Revenue in the US segment was $1.3 billion, an increase of 15% compared with the third quarter of 2023. Sales of companion animal products increased 18%, driven by the company's monoclonal antibody (mAb) products for osteoarthritis (OA) pain, Librela ® for dogs and Solensia ® for cats.
However, shares of Zoetis (NYSE:ZTS) traded lower yesterday after the FDA notified veterinarians about adverse events linked to the company’s pain therapy, Librela (bedinvetmab injection).
Conclusion:
The FDA has raised a safety concern regarding the canine osteoarthritis drug, Librela, and has issued a warning letter to veterinarians about the adverse events associated with this monoclonal antibody treatment. The FDA has advised that pet owners be informed of the potential side effects that may occur after administering Librela, which should already be occurring for any drug being administered to a pet. While the FDA cannot mandate changes, it has recommended that the product’s label include a “Post Approval Experience” section detailing the adverse events identified during its evaluation.
Meanwhile, Zoetis continues to benefit from marketing Librela as a “miracle cure” for pain, tapping into the emotional connection pet owners have with their animals.
As consumers, it is crucial that we remain proactive in educating veterinary professionals about the risks associated with this and other commercially available treatments.
What should a veterinarian do if a patient treated with Librela has an adverse event?
If a dog under your care experiences an adverse event while receiving Librela, the FDA encourages you to report it to Zoetis, the drug sponsor, at 1-888‑963-8471. Drug sponsors are required to submit reports of adverse drug events to FDA. If you prefer to report directly to FDA, please see www.fda.gov/reportanimalae.
When reporting adverse events to the FDA and/or Zoetis, please include, if available, a full medical history, how many times the dog has received Librela, and the lot number on the vial used.






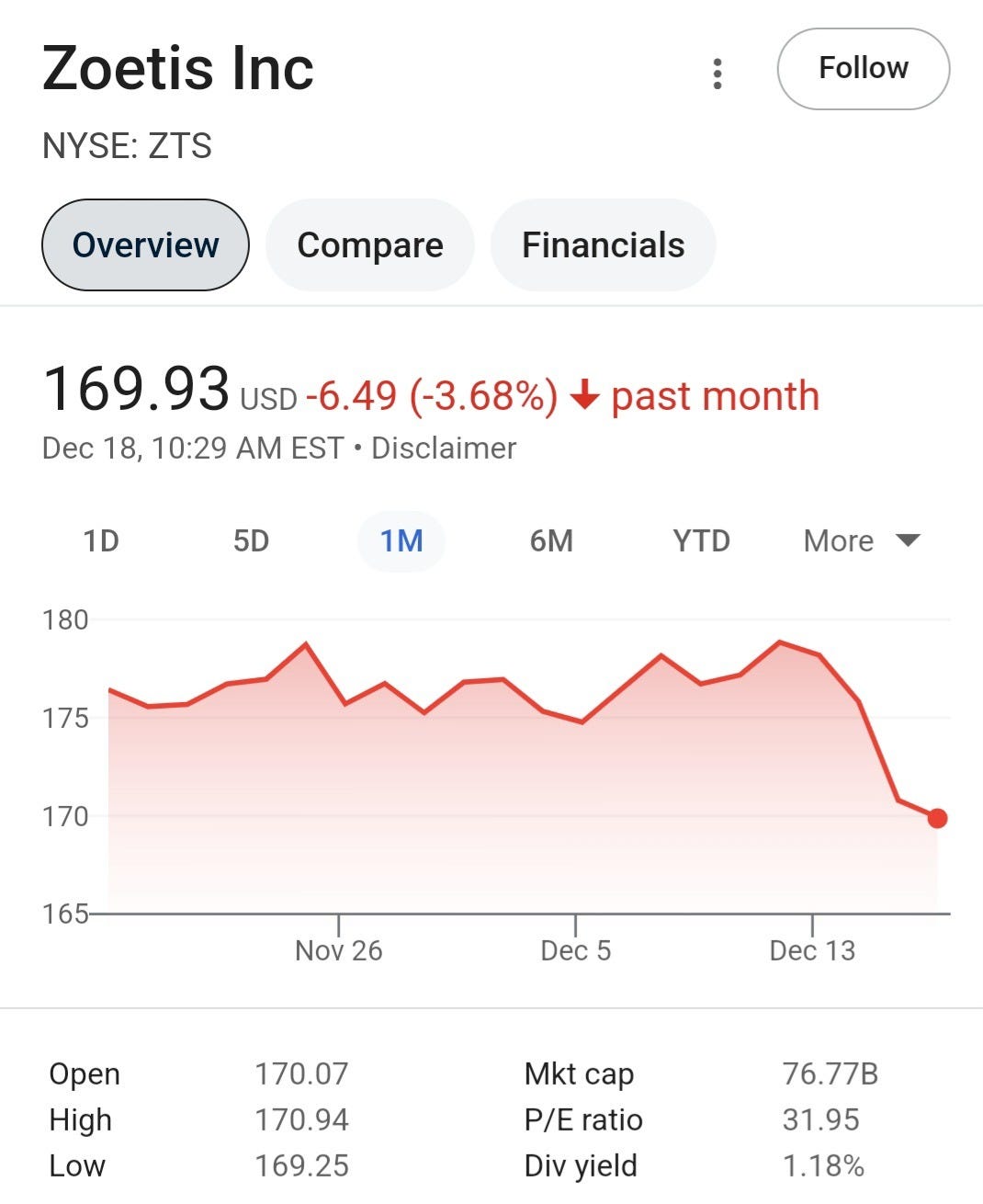
Notice that they just recite the mantra “safe and effective.” And the boldness to continue on even when the FDA is drawing attention to an actual safety signal. Which makes you wonder how this thing made it in the first place…like NGF inhibition is no joke. And if they are so inhumane as to keep this on the market…it is said that for those who mistreat animals so it goes for how people treat other people… “treating the pain associated with osteoarthritis” does NOT get to the root cause…just pain…along with a whole host of other side effects. There needs to be way better screening for even the drug targets just by themselves…there is a reason this was approved for veterinary…terrible.
check out my posts over the past year. Thanks for helping shine the light on this!